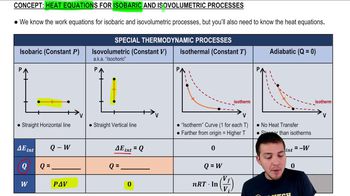
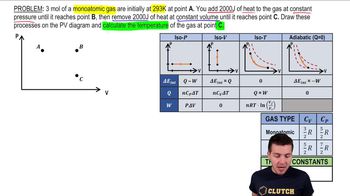
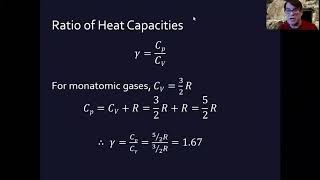
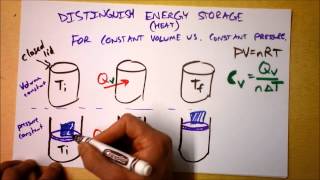
22. The First Law of Thermodynamics
Heat Equations for Special Processes & Molar Specific Heats
Problem 19e
Textbook Question
Textbook QuestionOn a warm summer day, a large mass of air (atmospheric pressure 1.01 * 10^5 Pa) is heated by the ground to 26.0°C and then begins to rise through the cooler surrounding air. (This can be treated approximately as an adiabatic process; why?) Calculate the temperature of the air mass when it has risen to a level at which atmospheric pressure is only 0.850 * 105 Pa. Assume that air is an ideal gas, with g = 1.40. (This rate of cooling for dry, rising air, corresponding to roughly 1 C° per 100 m of altitude, is called the dry adiabatic lapse rate.)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
457
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos