19. Fluid Mechanics
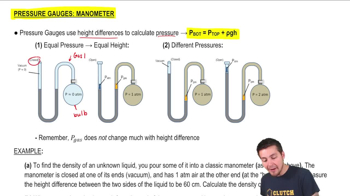
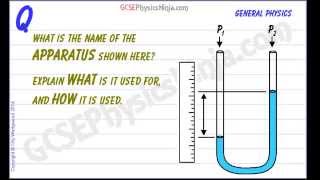
Pressure Gauge: Manometer
Multiple Choice
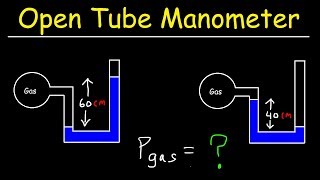
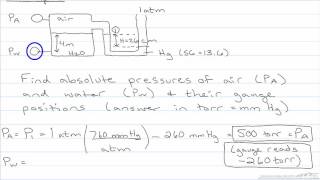
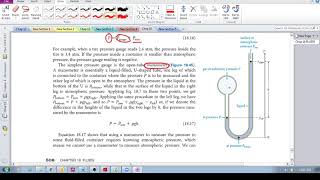
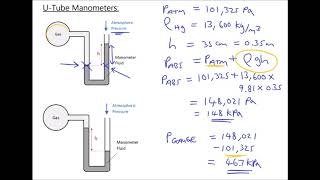
Multiple ChoiceA classic manometer (as shown below) has one of its ends open, and a 2 atm gas on the other. When mercury (13,600 kg/m3 ) is added to the manometer, you measure the top of the mercury column on the left to be 40 cm higher than the mercury column on the right. Calculate the atmospheric pressure that the manometer is exposed to, in units of atm. (Use g=9.8 m/s2.)
A
0.477 atm
B
1.0 atm
C
1.47 atm
D
2.53 atm
303
views
1
rank
Related Videos
Related Practice
Showing 1 of 9 videos