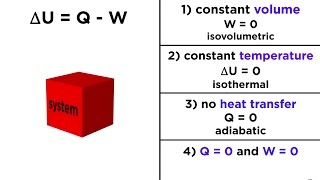
22. The First Law of Thermodynamics
First Law of Thermodynamics
Multiple Choice
Multiple ChoiceThe internal energy of a system decreases by 500 J, and 230 J of work is done on the system. What is the heat transfer into or out of this system?
A
730 J
B
270 J
C
– 270 J
D
– 730 J
425
views
3
rank
Related Videos
Related Practice
Showing 1 of 9 videos