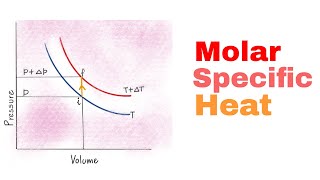
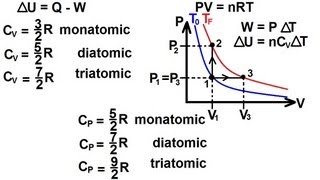
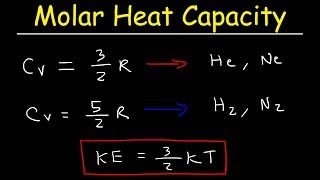
22. The First Law of Thermodynamics
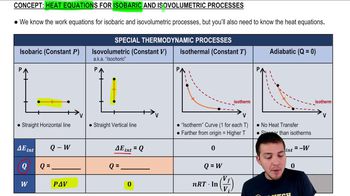
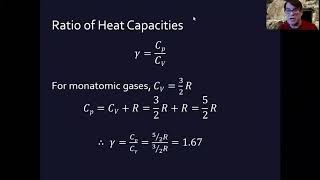
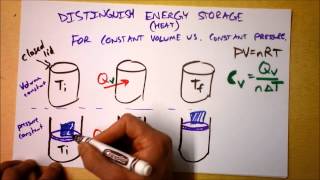
Heat Equations for Special Processes & Molar Specific Heats
Problem 19h
Textbook Question
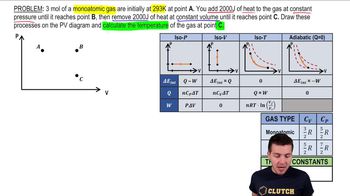
Textbook QuestionA monatomic ideal gas that is initially at 1.50 * 10^5 Pa and has a volume of 0.0800 m^3 is compressed adiabatically to a volume of 0.0400 m^3. (c) What is the ratio of the final temperature of the gas to its initial temperature? Is the gas heated or cooled by this compression?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
304
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos