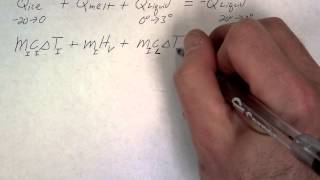20. Heat and Temperature
Calorimetry with Temperature and Phase Changes
Problem 17b
Textbook Question
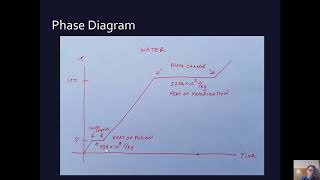
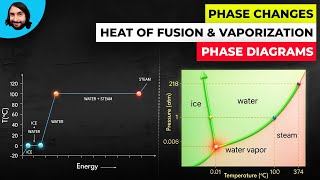
Textbook QuestionA 4.00-kg silver ingot is taken from a furnace, where its temperature is 750.0°C, and placed on a large block of ice at 0.0°C. Assuming that all the heat given up by the silver is used to melt the ice, how much ice is melted?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
377
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos