22. The First Law of Thermodynamics
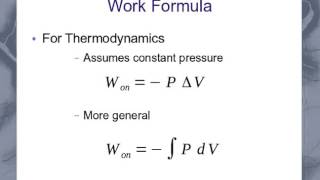
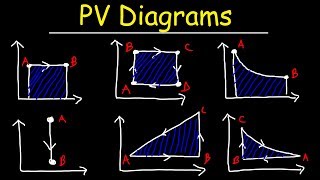
Work Done Through Multiple Processes
Multiple Choice
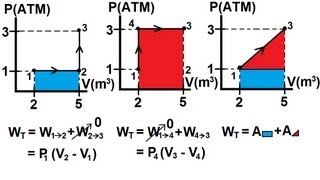
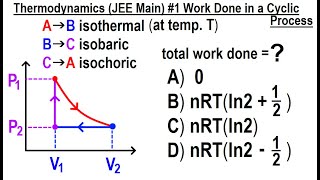
Multiple ChoiceA gas with an initial volume of 0.2 m3 is heated at constant volume, and the pressure increases from 2×105 Pa to 5×105. Then, it compresses at constant pressure until it reaches a final volume of 0.12 m3. Draw the two processes in the PV diagram below and find the total work done by the gas.
A
- 1.6 × 104 J
B
4 × 104 J
C
0 J
D
- 4 × 104 J
275
views
2
rank
Related Videos
Related Practice
Showing 1 of 8 videos