21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Problem 18q
Textbook Question
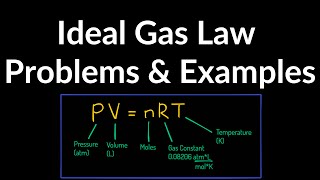
Textbook QuestionA cylindrical tank has a tight-fitting piston that allows the volume of the tank to be changed. The tank originally contains 0.110 m^3 of air at a pressure of 0.355 atm. The piston is slowly pulled out until the volume of the gas is increased to 0.390 m^3. If the temperature remains constant, what is the final value of the pressure?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
698
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos