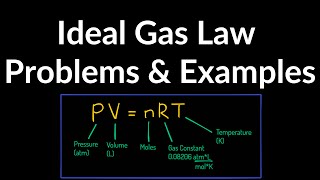
21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Problem 18h
Textbook Question
Textbook QuestionHow Close Together Are Gas Molecules? Consider an ideal gas at 27°C and 1.00 atm. To get some idea how close these molecules are to each other, on the average, imagine them to be uniformly spaced, with each molecule at the center of a small cube. (a) What is the length of an edge of each cube if adjacent cubes touch but do not overlap?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
278
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos