22. The First Law of Thermodynamics
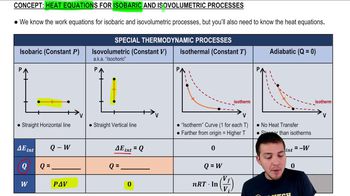
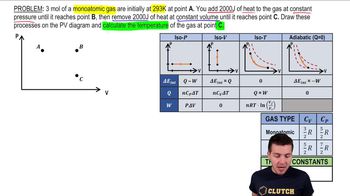
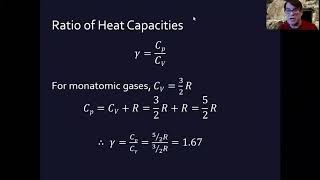
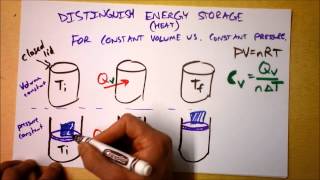
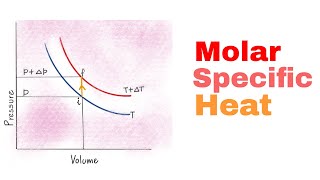
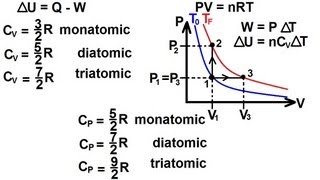
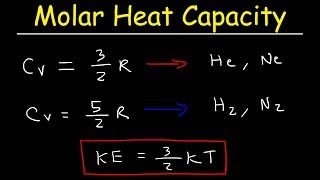
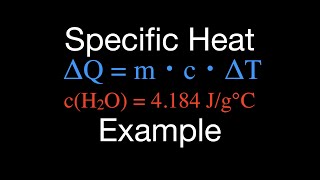
Heat Equations for Special Processes & Molar Specific Heats
Problem 19j
Textbook Question
Textbook QuestionFive moles of monatomic ideal gas have initial pressure 2.50 * 10^3 Pa and initial volume 2.10 m^3 . While undergoing an adiabatic expansion, the gas does 1480 J of work. What is the final pressure of the gas after the expansion?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
14mPlay a video:
961
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos