22. The First Law of Thermodynamics
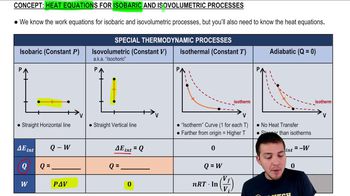
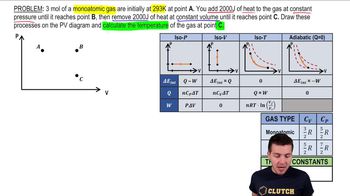
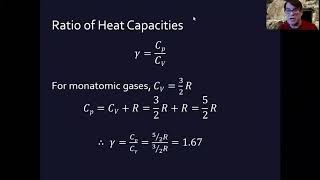
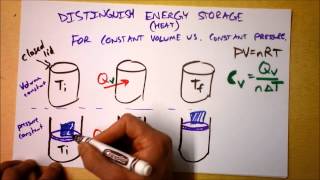
Heat Equations for Special Processes & Molar Specific Heats
Problem 19f
Textbook Question
Textbook QuestionA player bounces a basketball on the floor, compressing it to 80.0% of its original volume. The air (assume it is essentially N2 gas) inside the ball is originally at 20.0°C and 2.00 atm. The ball's inside diameter is 23.9 cm. (a) What temperature does the air in the ball reach at its maximum compression? Assume the compression is adiabatic and treat the gas as ideal.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
291
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos