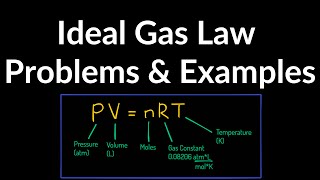
21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Problem 18j
Textbook Question
Textbook QuestionModern vacuum pumps make it easy to attain pressures of the order of 10^-13 atm in the laboratory. Consider a volume of air and treat the air as an ideal gas. (b) How many molecules would be present at the same temperature but at 1.00 atm instead?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
234
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos