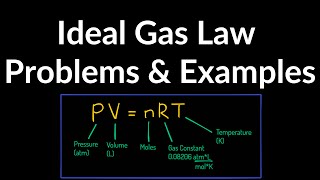
21. Kinetic Theory of Ideal Gases
The Ideal Gas Law
Problem 18b
Textbook Question
Textbook QuestionYou have several identical balloons. You experimentally determine that a balloon will break if its volume exceeds 0.900 L. The pressure of the gas inside the balloon equals air pressure (1.00 atm). (a) If the air inside the balloon is at a constant 22.0°C and behaves as an ideal gas, what mass of air can you blow into one of the balloons before it bursts?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
295
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos