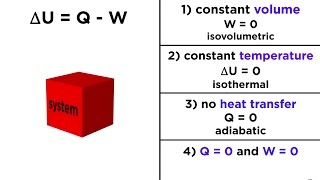
22. The First Law of Thermodynamics
First Law of Thermodynamics
Problem 19g
Textbook Question
Textbook QuestionBoiling Water at High Pressure. When water is boiled at a pressure of 2.00 atm, the heat of vaporization is 2.20 * 10^6 J/kg and the boiling point is 120°C. At this pressure, 1.00 kg of water has a volume of 1.00 * 10^-3 m^3 , and 1.00 kg of steam has a volume of 0.824 m^3. (a) Compute the work done when 1.00 kg of steam is formed at this temperature.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
5mPlay a video:
1290
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos