Back
BackpH Scale definitions
Terms in this set (9)
Base
A substance with a pH above 7 that decreases hydrogen ion concentration by accepting hydrogen ions from a solution.
pH
A measure of a solution's acidity or basicity, ranging from 0 to 14, where 7 is neutral, values below 7 are acidic, and values above 7 are basic.
Neutral Solution
A solution with a pH of 7, indicating equal concentrations of hydrogen ions (H⁺) and hydroxide ions (OH⁻).
Acid
A substance that donates hydrogen ions (H⁺) to a solution, resulting in a pH less than 7.
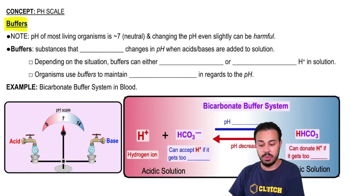
Buffer
A substance that maintains pH stability by accepting excess hydrogen ions in acidic solutions and donating hydrogen ions in basic solutions.
Hydroxide Ion
A negatively charged ion (OH⁻) formed when a base dissociates in water, increasing the solution's pH by reducing hydrogen ion concentration.
Concentration
The amount of a substance in a given volume of solution, often measured in moles per liter (M).
Acidic Solution
A solution with a pH less than 7, indicating a higher concentration of hydrogen ions.
Basic Solution
A solution with a pH above 7, characterized by a lower concentration of hydrogen ions and the ability to accept hydrogen ions from the solution.