Properties of Water- Cohesion and Adhesion exam Flashcards
 Back
BackTerms in this set (25)
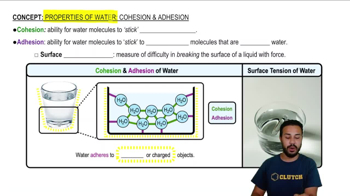
Cohesion
The ability for water molecules to stick together through hydrogen bonds.
Adhesion
The ability for water molecules to stick to other polar surfaces.
What is surface tension?
The resistance of a liquid's surface to disruption, created by cohesion and adhesion.
Hydrogen Bonds
Weak bonds that form between water molecules, contributing to cohesion.
What allows a paperclip to float on water?
Surface tension created by the cohesion and adhesion of water molecules.
Polarity
A characteristic of water molecules that allows them to form hydrogen bonds.
What does the term 'co' in cohesion signify?
Together
What type of surfaces does water adhere to?
Polar and/or charged surfaces.
Surface Tension
A measure of the difficulty in breaking the surface of a liquid with force.
Why does water not adhere to all molecules?
Water only adheres to specific types of molecules that are polar and/or charged.
What creates surface tension in water?
The cohesion and adhesion of water molecules.
What is an example of adhesion?
Water molecules sticking to glass.
What is an example of cohesion?
Water molecules sticking to other water molecules.
Why is understanding cohesion and adhesion important?
It is essential for grasping water's role in biological systems and its significance for life.
What happens when a paperclip breaks the surface tension of water?
It sinks to the bottom of the glass of water.
What is the significance of water's polarity?
It allows water to form hydrogen bonds, leading to cohesion and adhesion.
What role does surface tension play for certain organisms?
It allows them to exploit the surface of water, such as insects walking on water.
What is the root meaning of 'co' in cohesion?
Together
What is the relationship between cohesion and surface tension?
Cohesion contributes to the creation of surface tension in water.
What is the relationship between adhesion and surface tension?
Adhesion contributes to the creation of surface tension in water.
What is the effect of hydrogen bonds on water molecules?
They cause water molecules to stick together, demonstrating cohesion.
What is the effect of water's polarity on its properties?
It enables water to form hydrogen bonds, leading to cohesion and adhesion.
What happens when enough force is applied to the surface of water?
The surface tension is broken, and objects like a paperclip will sink.
Why is glass a good example of adhesion?
Because glass is a polar object, allowing water to adhere to it.
What is the significance of hydrogen bonds in water?
They are crucial for the cohesion and adhesion properties of water.