7. Gases
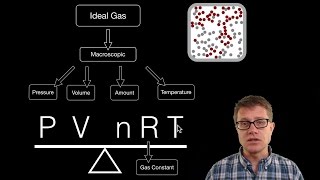
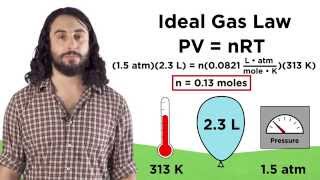
The Ideal Gas Law

Get help from an AI Tutor
Ask a question to get started.
Problem 45b
Textbook Question
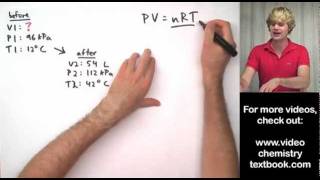
Textbook QuestionA piece of dry ice (solid carbon dioxide) with a mass of 28.8 g sublimes (converts from solid to gas) into a large balloon. Assuming that all of the carbon dioxide ends up in the balloon, what is the volume of the balloon at 22 °C and a pressure of 742 mmHg?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
7mPlay a video:
3839
views
2
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos