7. Gases
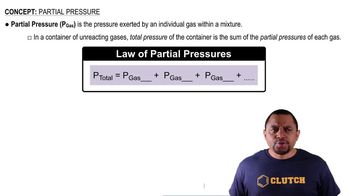
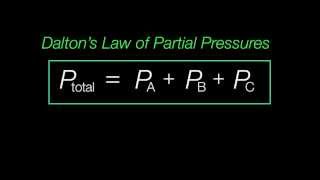
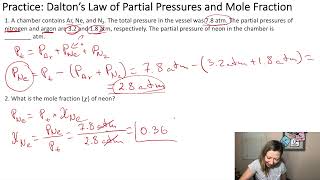
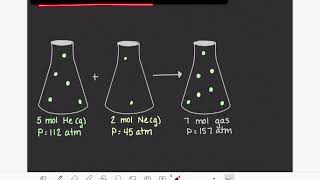
Partial Pressure

Get help from an AI Tutor
Ask a question to get started.
Problem 70
Textbook Question
Textbook QuestionA heliox deep-sea diving mixture contains 2.0 g of oxygen to every 98.0 g of helium. What is the partial pressure of oxygen when this mixture is delivered at a total pressure of 8.5 atm?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2881
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos