- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

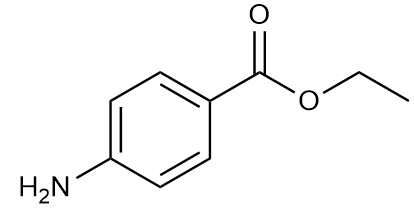
Benzocaine, a widely used local anesthetic, is often administered as benzocaine hydrochloride in clinical settings.
Why is benzocaine hydrochloride preferred over benzocaine?
Refer to the two dimethylamine molecules hydrogen-bonded with water shown below. Identify the bonds made or broken and where the electrons go when they react, forming an amine, an ammonium ion, OH–.

What is the equilibrium equation for the acid-base reaction of piperidine and water? Label each species as a base or acid.
Consider the following amines:
i. p-chloroaniline
ii. N-methylcyclopentylamine
iii. N-butylethylamine
What are the structures of the ammonium ion produced from the reaction of each of the following amines with an acid?
Consider the structure of 1,1-dimethylpyrrolidin-1-ium shown below:

Will 1,1-dimethylpyrrolidin-1-ium react with aqueous HBr? If so, identify the product.
Consider the following molecules:

List them in order of decreasing boiling point and justify your answer.
Determine if the amine shown below is soluble in water. Explain your answer.