15. Chemical Kinetics
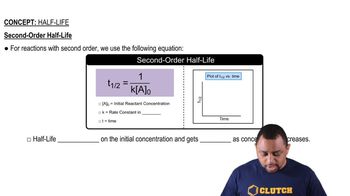
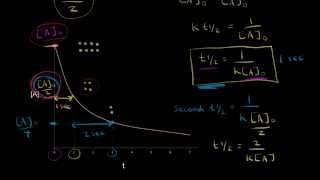
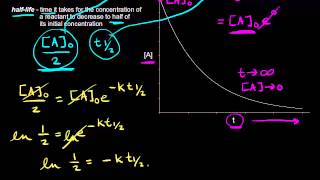
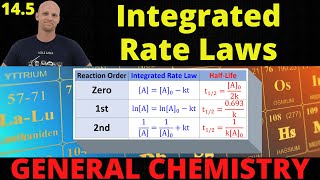
Half-Life

Get help from an AI Tutor
Ask a question to get started.
Problem 97b
Textbook Question
Textbook QuestionThe desorption (leaving of the surface) of a single molecular layer of n-butane from a single crystal of aluminum oxide is found to be first order with a rate constant of 0.128>s at 150 K. b. If the surface is initially completely covered with n-butane at 150 K, how long will it take for 25% of the molecules to desorb (leave the surface)? For 50% to desorb?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
473
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos