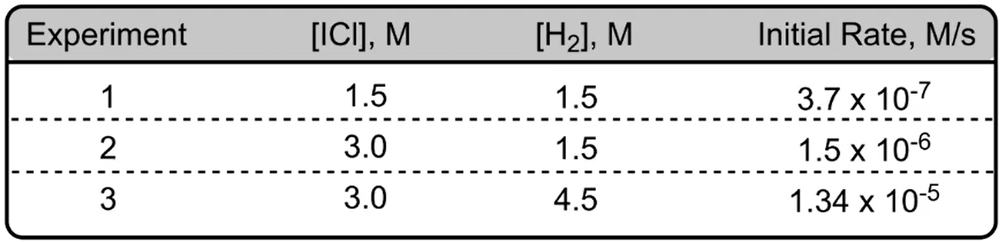
The rate law is a fundamental concept in chemical kinetics that expresses the relationship between the rate of a reaction and the concentrations of its reactants. It is represented by the equation:
Rate = k [A]x [B]y
In this equation, Rate refers to the speed at which the reaction occurs, k is the rate constant, and [A] and [B] are the concentrations of the reactants. The exponents x and y, known as reaction orders, indicate how the rate is affected by changes in the concentrations of the respective reactants.
It is important to note that the rate law focuses solely on the reactants and does not consider the concentrations of the products. This means that when determining the rate of a reaction, only the reactant concentrations are taken into account.
To determine the values of the reaction orders, one can utilize experimental data or a reaction mechanism, which outlines the step-by-step process of the reaction. While the specifics of reaction mechanisms are complex and will be explored in greater detail later, they play a crucial role in understanding how the rate law is formulated.
In summary, the rate law serves as a vital tool for connecting the changes in reactant concentrations, the rate constant, and the reaction orders to calculate the overall rate of a chemical reaction.