15. Chemical Kinetics
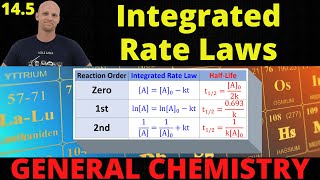
Integrated Rate Law

Get help from an AI Tutor
Ask a question to get started.
Problem 83c
Textbook Question
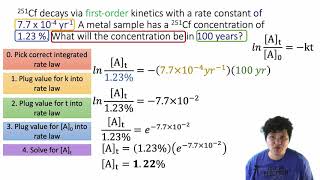
Textbook QuestionThe tabulated data were collected for this reaction at 500 °C: CH3CN(g)¡CH3NC( g) Time (h) [Ch3Cn] (M) 0.0 1.000 5.0 0.794 10.0 0.631 15.0 0.501 20.0 0.398 25.0 0.316 a. Determine the order of the reaction and the value of the rate constant at this temperature.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
1434
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos