1. Intro to General Chemistry
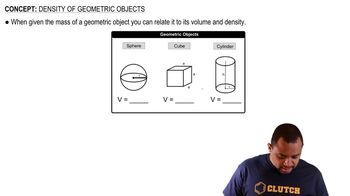
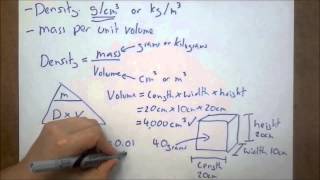
Density of Geometric Objects

Get help from an AI Tutor
Ask a question to get started.
Problem 107b
Textbook Question
Textbook QuestionCarbon-12 contains six protons and six neutrons. The radius of the nucleus is approximately 2.7 fm (femtometers) and the radius of the atom is approximately 70 pm (picometers). Calculate the volume of the nucleus and the volume of the atom.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
1154
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 10 videos