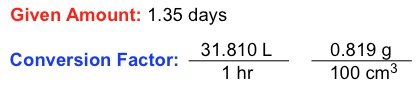
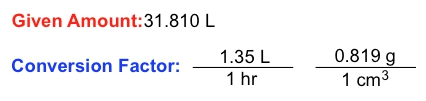
In the study of chemistry, dimensional analysis plays a crucial role in solving complex word problems by isolating specific units. A fundamental aspect of dimensional analysis is the conversion factor, which is a ratio or fraction that connects two different units. For instance, the relationship between days and hours can be expressed as a conversion factor: one day equals 24 hours. This can be represented as a fractional ratio, either as:
\[ \frac{1 \text{ day}}{24 \text{ hours}} \quad \text{or} \quad \frac{24 \text{ hours}}{1 \text{ day}} \]
This demonstrates how different units relate to one another. In addition to conversion factors, we also encounter given amounts, which are values expressed in a single unit. For example, if you study for 3 hours, the given amount is simply 3 hours, without any connection to other units.
Understanding how to identify and utilize conversion factors and given amounts is essential for mastering dimensional analysis. This skill will be particularly useful as you tackle more complex problems in chemistry. As you prepare for quizzes and exams, practice identifying these components in various questions to strengthen your analytical skills.









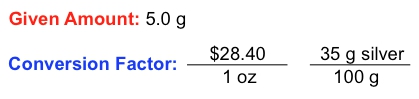





 2 students found this helpful
2 students found this helpful