15. Chemical Kinetics
Integrated Rate Law

Get help from an AI Tutor
Ask a question to get started.
Problem 131
Textbook Question
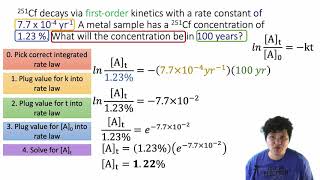
Textbook QuestionThe reaction 2 NO1g2 + O21g2S 2 NO21g2 has the thirdorder rate law rate = k3NO423O24, where k = 25 M-2 s-1. Under the condition that 3NO4 = 2 3O24, the integrated rate law is 13O242 = 8 kt +113O24022 What are the concentrations of NO, O2, and NO2 after 100.0 s if the initial concentrations are 3NO4 = 0.0200 M and 3O24 = 0.0100 M?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
174
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos