14. Solutions
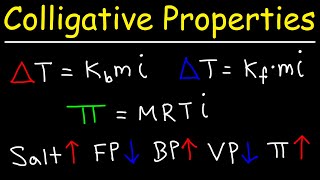
Vapor Pressure Lowering (Raoult's Law)

Get help from an AI Tutor
Ask a question to get started.
Problem 68
Textbook Question
Textbook QuestionAt 20 °C, the vapor pressure of benzene 1C6H62 is 75 torr, and that of toluene 1C7H82 is 22 torr. Assume that benzene and toluene form an ideal solution. (b) What is the mole fraction of benzene in the vapor above the solution described in part (a)?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1926
views
1
comments
Was this helpful?
Related Videos
Related Practice
Showing 1 of 7 videos