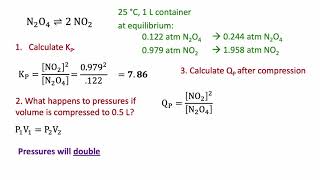
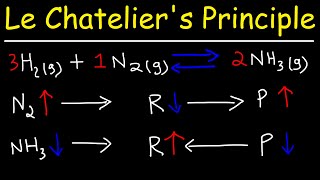
16. Chemical Equilibrium
Le Chatelier's Principle

Get help from an AI Tutor
Ask a question to get started.
Problem 131
Textbook Question
Textbook QuestionBaking soda (sodium bicarbonate) decomposes when it is heated: 2 NaHCO31s2 ∆ Na2CO31s2 + CO21g2 + H2O1g2 ΔH° = + 136 kJ Consider an equilibrium mixture of reactants and products in a closed container. How does the number of moles of CO2 change when the mixture is disturbed by the following: (b) Adding water vapor
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
847
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 13 videos