- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Identify the organic product of the hydrogenation reaction below:
Determine the plausible product of the addition reaction of the alkene given below. (Ignore stereochemistry.)
What is the product formed in the reaction below?

Identify whether the reaction below is an alcohol substitution, elimination, or oxidation.

Draw the major product for the following reaction of an alcohol

Identify the organic product of the reaction below:
Write the balanced chemical equation of the reaction of a carboxylic acid and an alcohol to form isobutyl propionate. (esterification). [Use condensed formula.]
Write the balanced chemical equation for the base-catalyzed saponification reaction of propyl benzoate with NaOH. [Use condensed structural formula.]
Draw the products of the following reaction of carboxylic acid.
Identify if the reaction below is a condensation or an acid-base reaction. Predict the products.
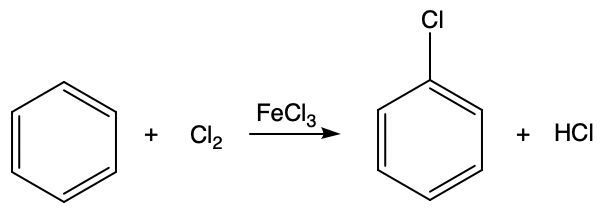
Identify if the following reaction involves alkane substitution, aromatic substitution, alcohol substitution, alkene addition, elimination, oxidation, or combustion.
Which element–S, Cl, Sn, or Ge –can be used as a semiconductor?
Determine the group 3A elements that have the highest and lowest melting points.
Tennessine is a synthetic element, therefore, very little empirical data on its properties is available. Is Tennessine (Ts) more likely to be a gas, a liquid, or a solid?
Identify the group of 8A element that can react with fluorine to form three different fluorides.
How does hydrogen exist in the below molecule: as H+, H-, or covalently bonded as an H atom?
HCl
Predict the products formed and provide the complete balanced equation for the following reaction:
C4H10(g)+H2O(g)catalyst→heat
Provide the complete balanced reaction equation for the following:
Ba(s) + H2O(l) →
Write a balanced chemical equation for the reaction of boron trifluoride (BF3) gas with water to form boric acid (H3BO3) and hydrogen fluoride (HF).
Identify whether the following statement is true or false. Boron is the most abundant element in the earth's crust among group 3A elements.