Which of the following statements is/are TRUE based on the figure below? Assume at a constant temperature.
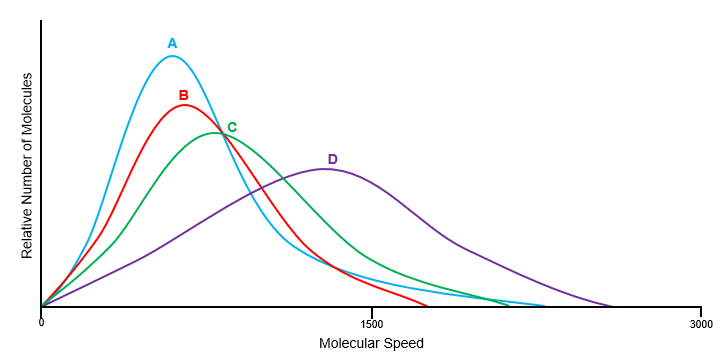
I) Gas D has the largest molar mass.
II) Gas B has a larger molar mass than gas C.
III) Every molecule of gas A is slower than every molecule of gas D.