- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Classify the following compound as an acid, base, or salt, and write its name: H2TeO3, whose polyatomic anion inhibits oxidation of a biological substrate.
Provide the distinction between a monoprotic acid and diprotic acid. Determine whether the acids listed below are monoprotic or diprotic:
(i) Hydrocyanic acid (HCN)
(ii) Tartaric acid (H2C4H4O6)
Identify which of the following representations is for a solution of strong Arrhenius monoprotic acid (HA) and which is for a solution of strong Arrhenius diprotic acid (H2A).
I.

II.

Give the balanced chemical equation that shows HCOOH as an acid according to the Arrhenius definition.
Explain what happens when benzoic acid (C6H5COOH) is dissolved in water. Note that benzoic acid is a weak acid.
Identify the statement that correctly describes the reaction
HCN (aq) + PH3(aq) → PH4+(aq) + CN- (aq)
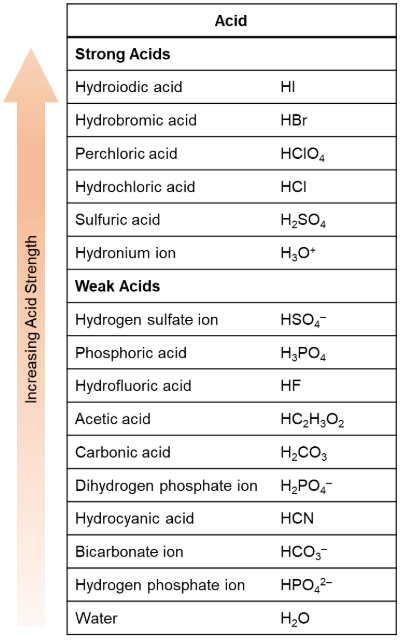
Using the table provided, determine which of the following acids is stronger: H2CO3 or HCl?
The following figure shows the electrostatic potential maps of the molecules CH3COOH and CF3COOH. Determine the most acidic hydrogen in each molecule. Compare their acidity and identify which is expected to be a stronger acid between the two.

The three acids are arranged in decreasing acid strength
HB > HA > HC
What is the decreasing order in terms of strength for their conjugate bases?
Derive an equation to solve for [H3O+] in an aqueous solution of hydrofluoric acid (HF). Hint: Start with Ka.
Rio Tinto is a highly acidic river in southwestern Spain. This river has an average pH of 2.00. What are the [H3O+] and the [OH−] of the river?
Provide the missing data for bleach in the following table:

At 25°C, An aqueous solution in a beaker has [OH-] = 8.97x 10-2M. Which statement about the solution is true?
I. The solution has [H3O+] = 1.11x10-13 M
II. The solution has a pH of 1.05
III. The solution is basic.