- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Phenols undergo aromatic substitution reactions. Write the chemical equation when p-nitrophenol reacts with Cl2. Note that this reaction will form two substitution products.
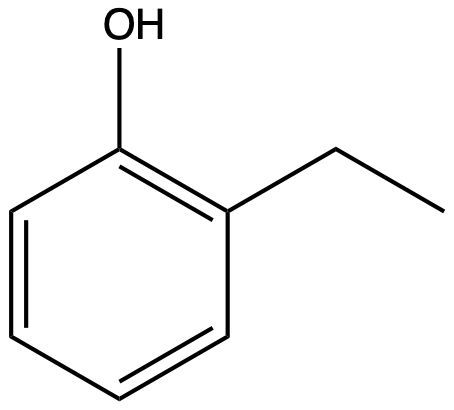
Determine the IUPAC name for this structure:
Sketch the line-angle formula of the following compound: cyclopentyl propyl ether
Which of the following generic structures represent a thiol and an alcohol?
Sketch the condensed structural formula of the product of the reaction shown below.

Determine whether the structure written in italics undergoes oxidation or reduction:
In a glycolysis step, the biomolecule NAD+ is converted into NADH.
The ester derivatives of the industrial chemical 'acid of butter' are widely used as food and perfume additives. The 'acid of butter' is also formed in the body when 1-butanol is completely metabolized. Facilitated by an enzyme, 1-butanol is converted to butanal, which is further converted to acid. If the 'acid of butter' is simply the oxidized form of 1-butanol, what is the structure of the acid?
The oxidation of the dithiol 2,6-dimethyl-3,5-heptanedithiol produces a 5-membered ring including a disulfide group as part of the ring. Sketch the line structure of the product.
Consider the following steroid hormone:
Identify the functional groups of (i) and (ii).
Sketch the following aldehyde or ketone compound: 3-ethyl-4-methylhexanal
Which can be oxidized into carboxylic acids: aldehydes, or ketones?
What tests differentiate a sample of an aldehyde from a sample of a ketone?
Draw the alcohol product of the given reaction using a condensed structural formula:
reduction of propionaldehyde by hydrogen in the presence of a platinum catalyst
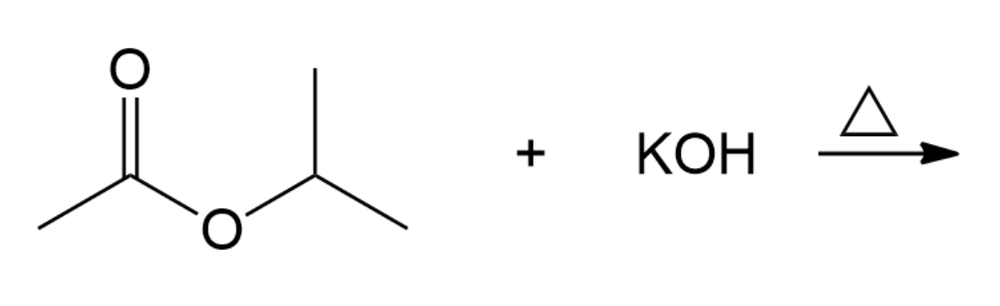
Consider the reaction shown below.

What is the structure of the hemiacetal or hemiketal that will be produced?
Draw a plausible condensed structural formula for 3,3-dichloropropanoic acid.
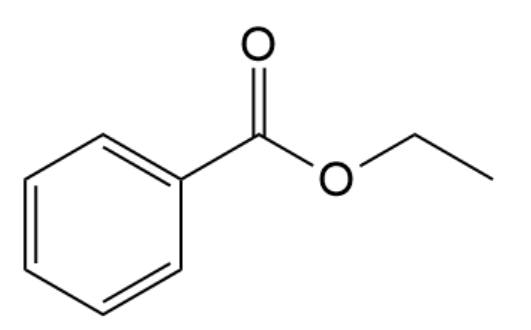
Give the common name (if any) and IUPAC name for the compound below:
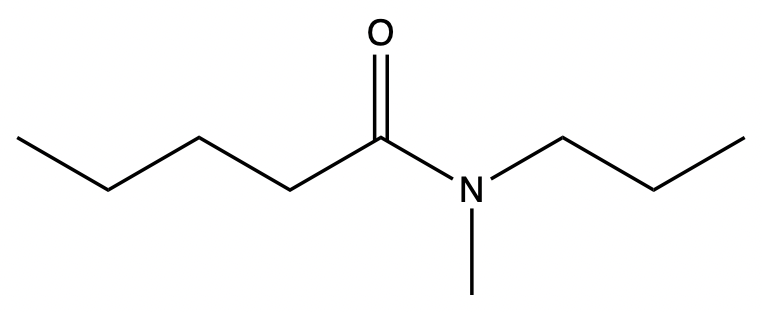
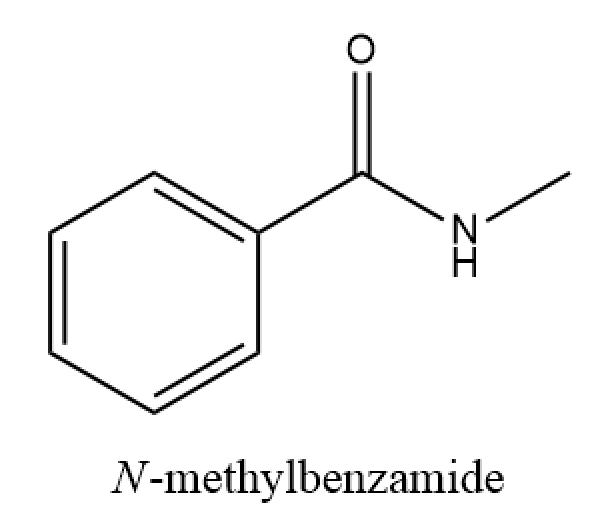
Determine the IUPAC name of the given amide.
Write a balanced chemical equation showing the ionization of hexanoic acid in water.
Propyl propanoate is used in the fragrance industry for its fruity odor. Determine the name of the carboxylic acid and alcohol involved in its synthesis.
Write a balanced chemical equation for the acid hydrolysis of propyl acetate, a flavoring agent in food.
Illustrate the products of the following reaction:
Draw a plausible structure of the amide product in the given reaction.
Draw the structure of the products produced when the amide shown below is hydrolyzed with HCl.