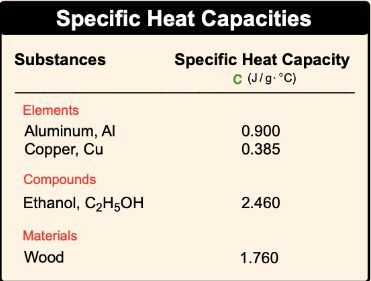
Heat capacity is a fundamental concept in thermodynamics that describes how a substance responds to the application of heat. When heat is added to an object, its temperature increases, demonstrating a direct relationship between the amount of heat applied and the resulting temperature change. This relationship can be expressed mathematically as:
\[ q \propto \Delta T \]
In this equation, q represents the heat added to the substance, and ΔT denotes the change in temperature. The proportionality indicates that as the heat (q) increases, the temperature change (ΔT) also increases, assuming the substance's properties remain constant. Understanding this relationship is crucial for calculating heat capacity, which is defined as the amount of heat required to change the temperature of a given mass of a substance by one degree Celsius (or Kelvin).