- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

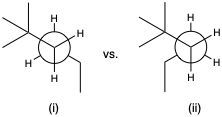
Identify the more stable conformation. If they are equally stable, write "no difference".
The chair conformation of bromocyclohexane with the bromo substituent in an axial position is about 0.43 kcal/mol more stable compared to the conformation with the bromo substituent in an equatorial position. Determine how much more stable the anti-conformation of 1-bromopropane is than its gauche conformation. (Rotate about C1-C2 bond)
Construct a potential energy graph for n-pentane as it rotates about the bond between C2 and C3. Start with a 0° dihedral angle and rotate the front carbon.
Table:

Draw potential energy vs dihedral angle graph for conformational analysis of propane as it rotates around the single bond between C1 and C2. Also, draw the Newman projections for each eclipsed and staggered conformation and show the dihedral angles.
Hint: One C—H bond eclipsed with another C—H bond contributes to 4.2 KJ/mol torsional energy. One C—H bond eclipsed with a C—CH3 bond contributes to 5.4 KJ/mol torsional energy.
Based on the given pair of conformations, predict which conformation is expected to be more favored at equilibrium.

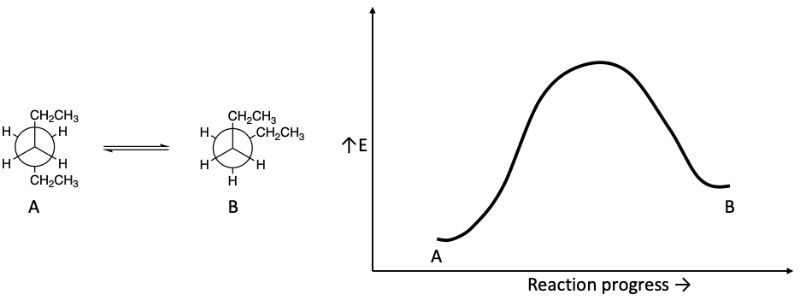
A reaction coordinate diagram is a plot of potential energy versus the progress of a reaction. Which structure is located at the top of the hill if the given reaction coordinate diagram shows the conversion of conformation A to conformation B?
Determine structures that correspond to the same compound. Also identify structures that correspond to different compounds.