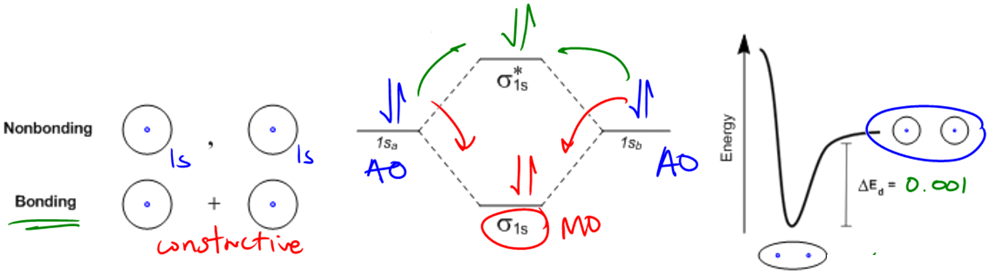
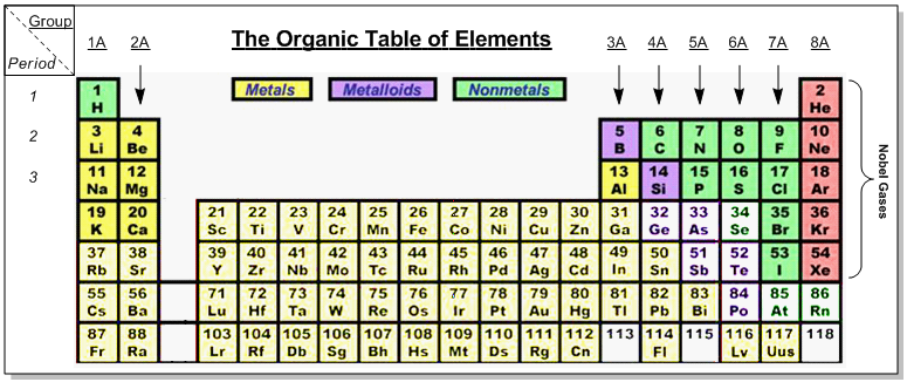
The octet rule is a fundamental principle in organic chemistry that explains the stability of atoms based on their electron configuration. Atoms achieve maximum stability when they have a full outer electron shell, typically consisting of eight electrons, which is referred to as the noble gas configuration. This configuration is crucial because it allows atoms to minimize their energy and maximize their stability.
In the periodic table, noble gases, located in group 8A, naturally possess a complete outer shell, making them chemically inert. Other elements strive to attain this stable configuration by either losing or gaining electrons. This behavior is encapsulated in the octet rule, which states that atoms tend to form bonds in such a way that they have eight electrons in their valence shell.
Understanding the octet rule is essential for predicting how different elements will interact and bond with one another. For instance, metals typically lose electrons to achieve a stable configuration, while nonmetals tend to gain or share electrons. This tendency to achieve a full outer shell drives the formation of ionic and covalent bonds, which are foundational concepts in chemistry.