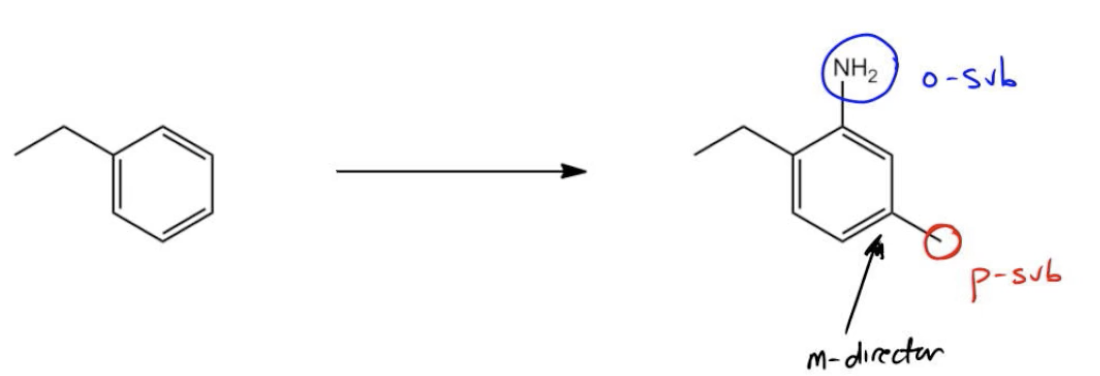
In the context of aromatic synthesis, transforming benzene or its derivatives into more complex molecules requires a solid understanding of the directing effects of substituents and the sequence of reactions involved. A key concept is the distinction between ortho/para and meta directors, which influence the position of new substituents added to the aromatic ring.
For instance, when starting with acetophenone, which is a ketone derivative of benzene, the goal may be to synthesize a target molecule that includes both a nitro group and a carboxylic acid (benzoic acid). The challenge lies in the fact that acetophenone acts as a meta director due to the electron-withdrawing nature of the carbonyl group. This means that if you were to nitrate acetophenone directly, the nitro group would be added in the meta position rather than the desired para position.
To achieve the desired substitution pattern, one must first convert the ketone into a substituent that can direct further reactions appropriately. This often involves a series of transformations. For example, one strategy could be to first convert the acetophenone into an ortho/para directing group, such as a hydroxyl group, through a reduction or substitution reaction. Once the ortho/para director is in place, nitration can be performed to add the nitro group in the para position.
After introducing the nitro group, the next step would be to convert the ketone into a carboxylic acid. This can typically be achieved through oxidation reactions, which will transform the ketone functional group into a carboxylic acid while maintaining the nitro group in the desired position.
In summary, the synthesis of complex aromatic compounds from simpler precursors like acetophenone involves careful planning of the reaction sequence, taking into account the directing effects of substituents and the transformations required to achieve the target structure. Understanding these principles allows for the strategic addition of functional groups in the desired positions on the aromatic ring.