6. Chemical Quantities & Aqueous Reactions
Balancing Redox Reactions: Basic Solutions

Get help from an AI Tutor
Ask a question to get started.
Problem 26
Textbook Question
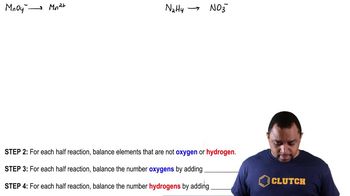
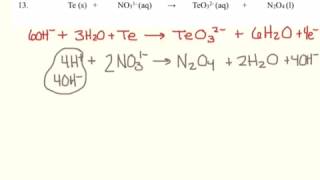
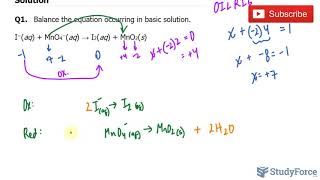
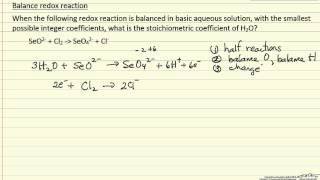
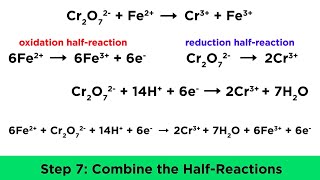
Textbook QuestionComplete and balance the following equations, and identify the oxidizing and reducing agents. (Recall that the O atoms in hydrogen peroxide, H2O2, have an atypical oxidation state.) (f) H2O21aq2 + ClO21aq2 ¡ ClO2-1aq2 + O21g2 (basic solution)
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
8mPlay a video:
330
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos