7. Gases
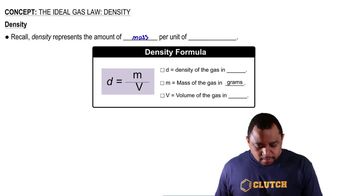
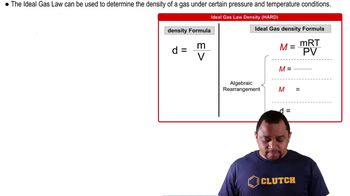
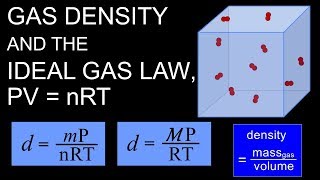
The Ideal Gas Law: Density

Get help from an AI Tutor
Ask a question to get started.
Problem 102
Textbook Question
Textbook QuestionPropane, C3H8, liquefies under modest pressure, allowing a large amount to be stored in a container. (a) Calculate the number of moles of propane gas in a 20-L container at 709.3 kPa and 25 C. (b) Calculate the number of moles of liquid propane that can be stored in the same volume if the density of the liquid is 0.590 g/mL. (c) Calculate the ratio of the number of moles of liquid to moles of gas. Discuss this ratio in light of the kinetic-molecular theory of gases.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
6mPlay a video:
922
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos