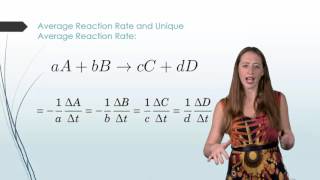15. Chemical Kinetics
Average Rate of Reaction

Get help from an AI Tutor
Ask a question to get started.
Problem 21
Textbook Question
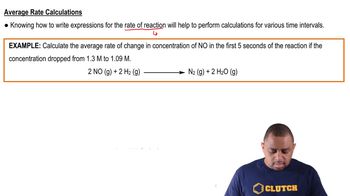
Textbook QuestionThe isomerization of methyl isonitrile 1CH3NC2 to acetonitrile 1CH3CN2 was studied in the gas phase at 215 C, and the following data were obtained: Time (s) 3CH3nC4 1M2 0 0.0165 2000 0.0110 5000 0.00591 8000 0.00314 12,000 0.00137 15,000 0.00074 (b) Calculate the average rate of reaction over the entire time of the data from t = 0 to t = 15,000 s.

 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
376
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 8 videos