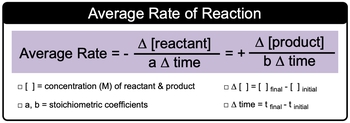
The average or general rate of a chemical reaction is defined as the change in concentration, measured in molarity, of a reactant or product over a specified period of time. It is important to note that the change in concentration for reactants is represented as a negative value, while for products, it is positive. This distinction arises because, during a reaction, reactants are consumed to form products, leading to a decrease in reactant concentration and an increase in product concentration over time.
The average rate can be expressed mathematically as:
Rate = \(\frac{\Delta [C]}{\Delta t}\)
In this equation, \(\Delta [C]\) represents the change in concentration, and \(\Delta t\) denotes the change in time. When applying this concept to a balanced chemical equation, the expression becomes more specific. For reactants, the change in concentration is calculated as:
\(\Delta [A] = \frac{[A]_{final} - [A]_{initial}}{a \cdot \Delta t}\)
Here, \([A]\) denotes the concentration of the reactant, and \(a\) is the stoichiometric coefficient from the balanced equation. Conversely, for products, the rate of formation is expressed as:
Rate = \(\frac{\Delta [B]}{b \cdot \Delta t}\)
In this case, \([B]\) represents the concentration of the product, and \(b\) is the corresponding stoichiometric coefficient. The change in product concentration is similarly calculated as:
\(\Delta [B] = \frac{[B]_{final} - [B]_{initial}}{b \cdot \Delta t}\)
Understanding these average rate expressions is crucial when analyzing the relationship between the rates of reactants and products in a balanced chemical equation. As we delve deeper into the topic, these concepts will help clarify how changes in the concentration of reactant molecules lead to the formation of products.