8. Thermochemistry
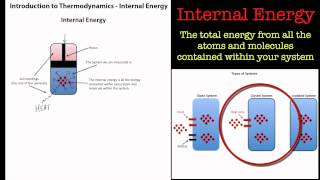
Internal Energy

Get help from an AI Tutor
Ask a question to get started.
Problem 38
Textbook Question
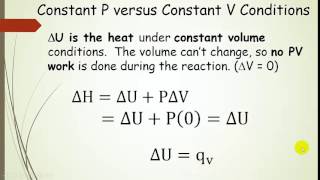
Textbook QuestionA gas is confined to a cylinder under constant atmospheric pressure, as illustrated in Figure 5.4. When 0.49 kJ of heat is added to the gas, it expands and does 214 J of work on the surroundings. What are the values of H and E for this process?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
0m:0sPlay a video:
2694
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 11 videos