In the study of thermodynamics, understanding how to determine the enthalpy change, or heat of reaction (ΔH), is crucial. There are three primary methods to calculate ΔH: constant volume calorimetry, thermochemical equations, and Hess's law. Constant volume calorimetry employs a bomb calorimeter specifically for combustion reactions, allowing for precise measurement of heat changes. Thermochemical equations utilize stoichiometry and balanced chemical equations to derive ΔH, while Hess's law enables the calculation of ΔH for an overall reaction by summing the enthalpies of individual partial reactions.
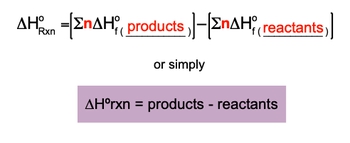
When these methods are not available, the standard enthalpy of formation can be used to find ΔH. It is important to note that the enthalpy of formation for an element in its standard state is defined as zero. The formula for calculating the standard enthalpy of reaction is expressed as:
\[\Delta H^\circ_{reaction} = \sum \Delta H_f^\circ (products) - \sum \Delta H_f^\circ (reactants)\]
This equation indicates that the standard enthalpy of reaction (ΔH°) is the difference between the total enthalpies of formation of the products and the reactants. The symbol Σ represents the summation of the enthalpies, while n denotes the moles of the substances involved. The standard enthalpy of formation (ΔH_f°) is measured in kilojoules per mole (kJ/mol) or sometimes in joules per mole (J/mol), depending on the units used.
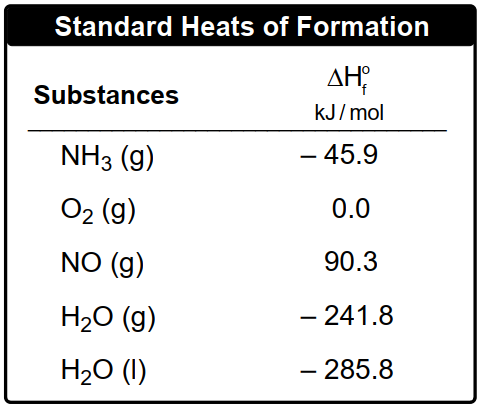
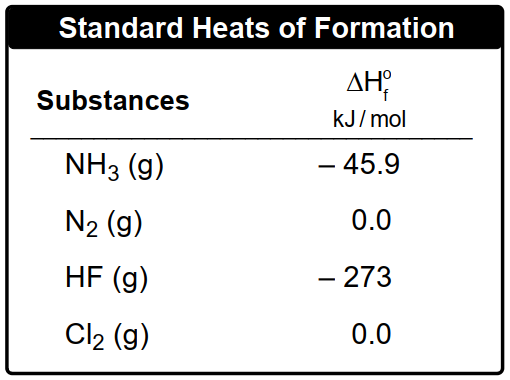
By applying this formula, one can effectively calculate the heat of reaction for various chemical processes, setting the stage for deeper exploration of thermodynamic principles in subsequent examples.