1. Intro to General Chemistry
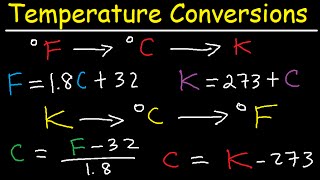
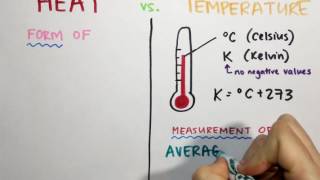
Temperature

Get help from an AI Tutor
Ask a question to get started.
Problem 61
Textbook Question
Textbook QuestionA 125 mL sample of water at 293.2 K was heated for 8 min, 25 s so as to give a constant temperature increase of 3.0 °F/min. What is the final temperature of the water in degrees Celsius?
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
523
views
1
rank
Was this helpful?
Related Videos
Related Practice
Showing 1 of 12 videos