Understanding the distinction between temperature and heat is essential in the study of thermal energy. While these terms are often used interchangeably, they represent different concepts. Thermal energy refers to the total kinetic and potential energies of all atoms within an object. Kinetic energy is the energy of motion, which relates to the movement of molecules and atoms, while potential energy pertains to the position of these molecules, such as their height above the ground.
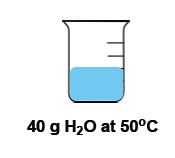
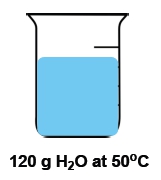
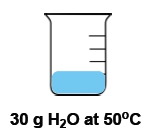
Temperature is defined as the average kinetic energy of the particles in a substance, serving as a measurement of thermal energy. It is an intensive property, meaning it does not depend on the amount of substance present. For instance, a cup of water and a gallon of water can both be at 100 degrees Celsius, yet the temperature remains the same regardless of the volume.
In contrast, heat is the transfer of thermal energy from an object at a higher temperature to one at a lower temperature. This flow occurs naturally, as heat moves from hotter to colder objects until thermal equilibrium is reached. Heat is considered an extensive property, as it is dependent on the quantity of the substance involved.
To illustrate this difference, consider two cubes: one with energetic, bouncing red balls representing higher temperature, and another with less movement indicating lower temperature. The wavy lines between the cubes symbolize the flow of heat from the hotter cube to the cooler one. This example reinforces that temperature is a specific measurement (e.g., 100 degrees, 20 degrees, or 98 degrees Fahrenheit), while heat is the process of energy transfer.
Recognizing the differences between intensive properties like temperature and extensive properties like heat is crucial for a deeper understanding of thermal dynamics and energy transfer processes.