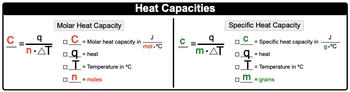
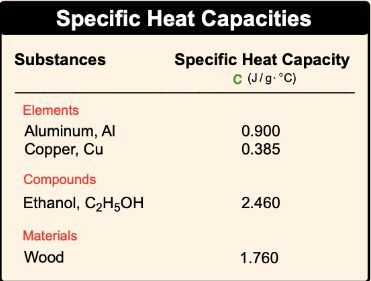
Heat capacity is a fundamental concept in thermodynamics that describes how a substance responds to the application of heat. When heat is added to an object, its temperature increases, demonstrating a direct relationship between heat and temperature change. This relationship can be expressed mathematically as:
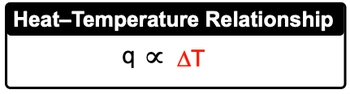
\[ q \propto \Delta T \]
In this equation, q represents the amount of heat added, and ΔT signifies the change in temperature. The greater the amount of heat applied to a substance, the larger the resulting temperature change will be. Understanding this principle is crucial for analyzing thermal processes and the behavior of materials under varying thermal conditions.