13. Liquids, Solids & Intermolecular Forces
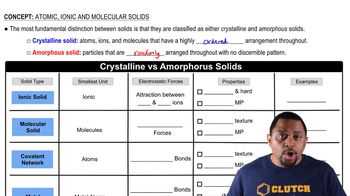
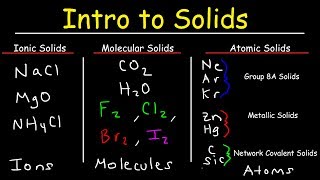
Atomic, Ionic, and Molecular Solids

Get help from an AI Tutor
Ask a question to get started.
Problem 103
Textbook Question
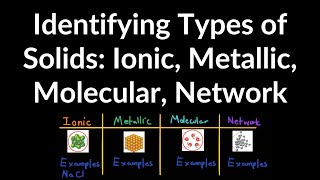
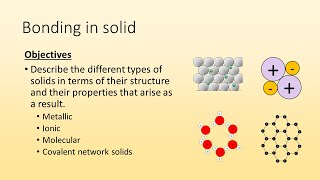
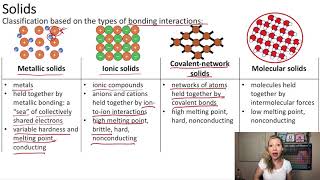
Textbook QuestionSelected chlorides have the following melting points: NaCl (801 °C), MgCl2 (714 °C), PCl3 (-94 °C), SCl2 (-121 °C) (a) For each compound, indicate what type its solid form is (molecular, metallic, ionic, or covalent-network).
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
1mPlay a video:
1374
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos