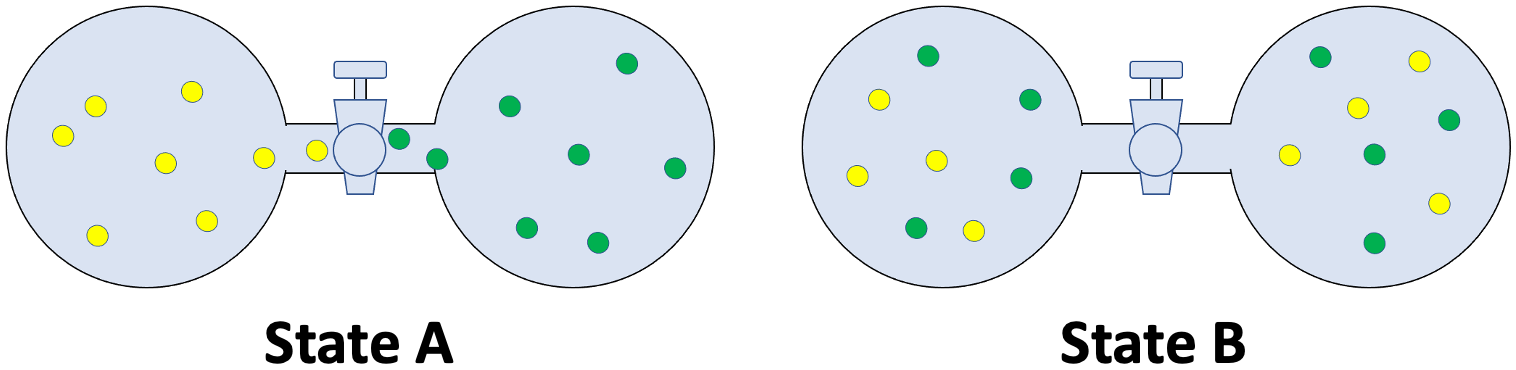
The spontaneous (ΔG < 0) process of going from State A to State B can be represented by the decreasing (from left to right) curve left side of the minimum/lowest point of the graph. Going from State B to another new state can be represented by the increasing (from left to right) curve right side of the minimum/lowest point of the graph since this process is non-spontaneous (ΔG > 0).