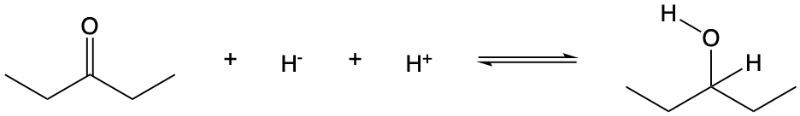- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Provide the line structure of the following compound:
Indicate whether it has a ketone functional group, an aldehyde functional group, or neither.
Give the systematic, IUPAC names and draw the line structures for the following compounds.
i.

ii.

iii.

iv. Diethyl ketone
Sketch the line structure of the molecule that satisfies the condition: cyclic ketone with 7 carbons and an ethyl group on the gamma carbon
Sketch the structures based on the compound names below:
i. pentanal
ii. butyl phenyl ketone
iii. 2,3,4-trimethylpentanal
iv. ethyl isopropyl ketone
Which is the correct common name of the given molecule?

Provide the correct condensed structural formula (if straight chain) or line-angle formula (if cyclic) of the compound 3-ethylbenzaldehyde.
Consider Compound X, an unbranched primary alcohol with a molecular formula of C4H10O. When Compound X undergoes a dehydration reaction (heated to 180° with an H2SO4 catalyst), Compound Y (C4H8) is produced. On the other hand, when Compound X undergoes an oxidation reaction, Compound Z (C4H8O) is formed. Based on the information given, predict the condensed structural formulas of Compounds X, Y, and Z and provide their IUPAC names.
Which can be oxidized into carboxylic acids: aldehydes, or ketones?
What tests differentiate a sample of an aldehyde from a sample of a ketone?
Identify the compound/s that will yield a positive result when subjected to Tollens' test.
i. 4-methyl-1-pentanol
ii. 4-methyl-2-pentanone
iii. 4-methylpentanal
The addition of a hydride ion (H-) and a proton (H+) results in the reduction of a carbonyl group. The elimination of H-and H+from an alcohol results in the formation of a carbonyl group.
Consider the following reaction:
True or False. The forward direction represents the reduction, and the reverse direction represents the oxidation.
Provide the ketone or aldehyde that was reduced to produce the alcohols given below:
i.

ii.

iii.

Provide the alcohol product of the following reaction using a condensed structural formula:
reduction of 3-methyl-4-heptanone by hydrogen in the presence of a palladium catalyst
Consider the molecule shown below.

Classify the molecule as acetal or ketal and provide the aldehyde or ketone that produced the molecule.