- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

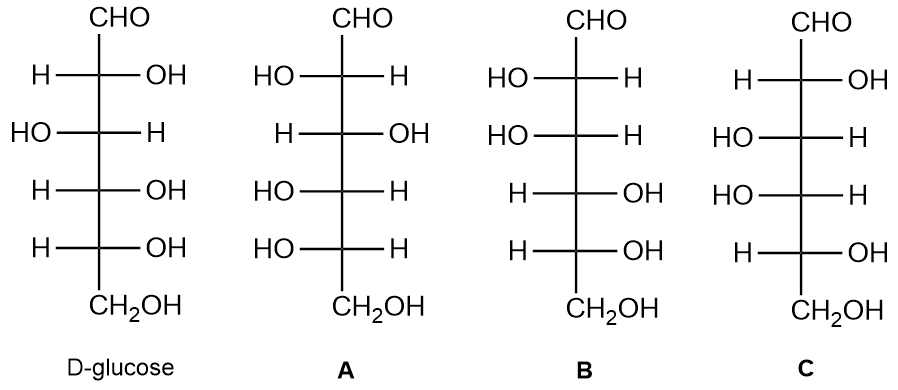
Consider the following structures and identify whether each of the structures A, B, and C is a diastereomer or an enantiomer of D-glucose.
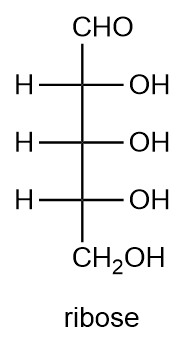
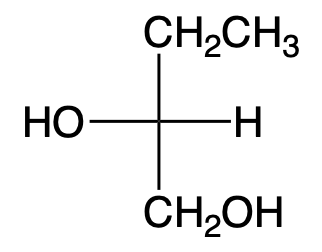
Draw the enantiomer of the given sugar. Describe the original sugar and its enantiomer as D- and L-sugars.
Classify the following as having either D or L configuration.
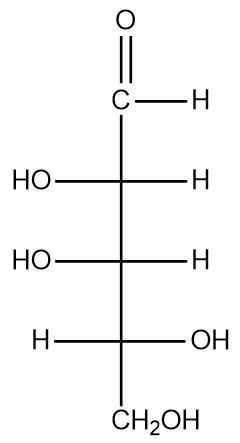
Determine whether the following carbohydrate is a D- or L-enantiomer.
Classify the pairs of compounds listed below as enantiomers, diastereomers, or anomers.
i. α-lactose and β-lactose
ii. D-idose and L-idose
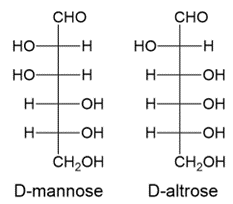
iii. D-mannose and D-altrose
D-glucose is a monosaccharide found in many fruits. Draw D-glucose in its cyclic hemiacetal form. [Both α and β anomers]
Provide the structure of both anomers of the monosaccharide shown below in pyranose ring form.
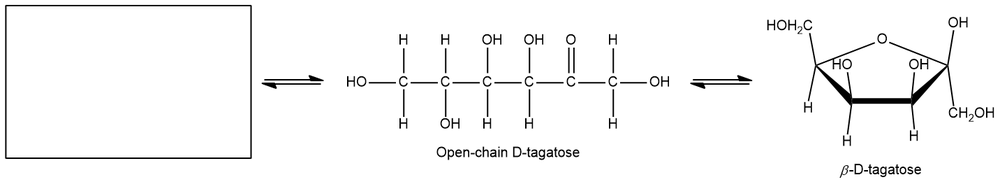
Provide the missing structure in the mutarotation rotation reaction of D-tagatose given below.
When D-mannose is treated with a reducing agent, it yields mannitol. Draw the structure of mannitol.
Volemitol is a naturally occurring seven-carbon sugar alcohol found in plants, red algae, fungi, mosses, and lichens. Volemitol is formed when D-manno-heptose undergoes reduction at carbon 1. Based on this information, draw the structure of volemitol.
If Benedict's test is used on the following carbohydrates, which will show positive results?
I. sucrose
II. lactose
III. D-mannose
IV. D-galactose