- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

What is the oxidation state of palladium in (C2H5CO2)2Pd?
Identify the reactions, among those listed below, that involve the oxidation of an organic molecule. Support your answer with a brief explanation.

For each reaction below, determine whether it is an oxidation, a reduction, or neither.

What is the structure of the compound with a molecular formula of C4H8O2 that has the following 1H NMR and IR spectra?
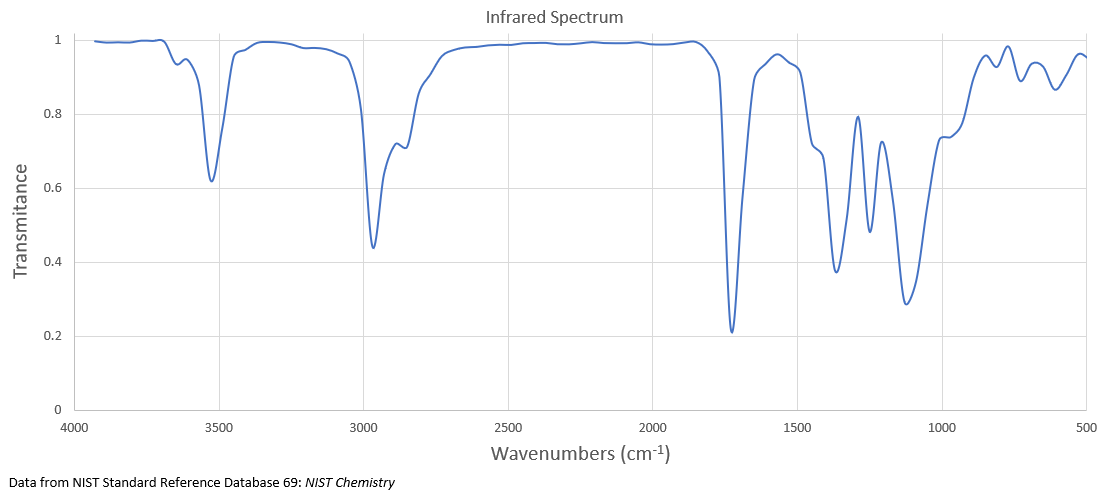

The IR spectrum of compound A with the molecular formula C3H6O is shown below.
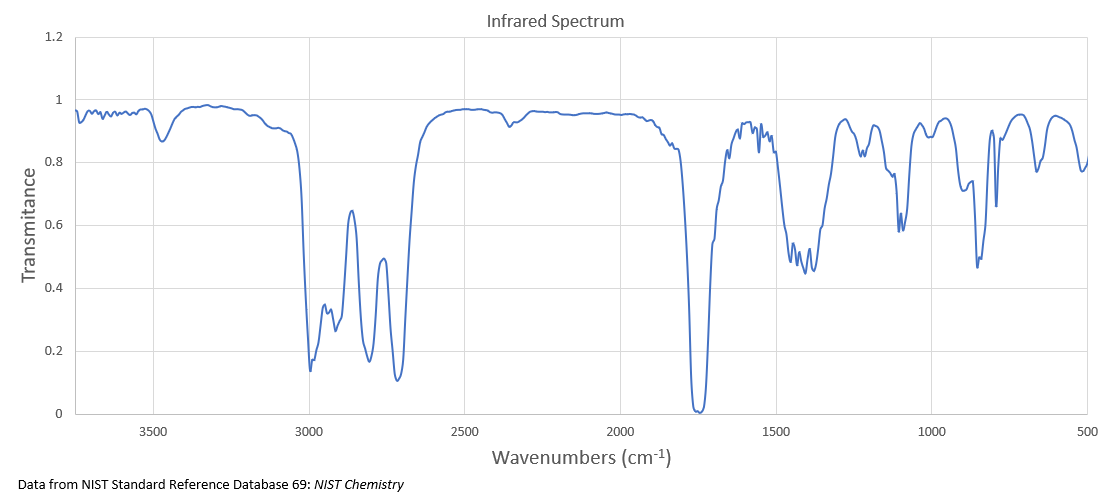
It undergoes reduction to produce compound B whose 1H NMR spectrum is displayed below.
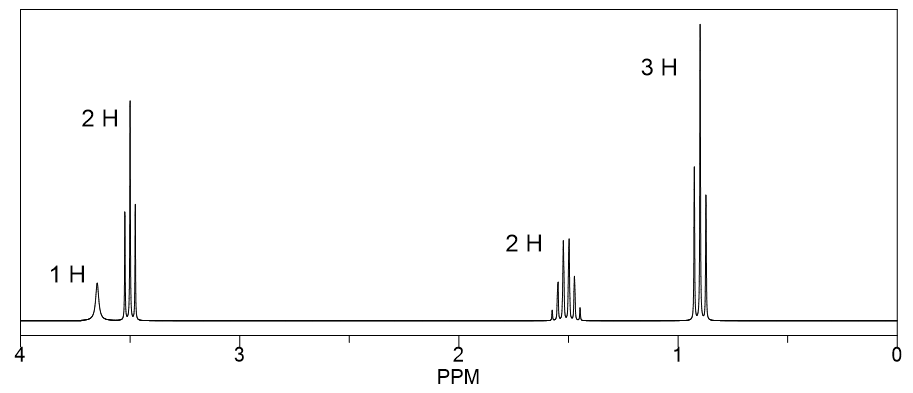
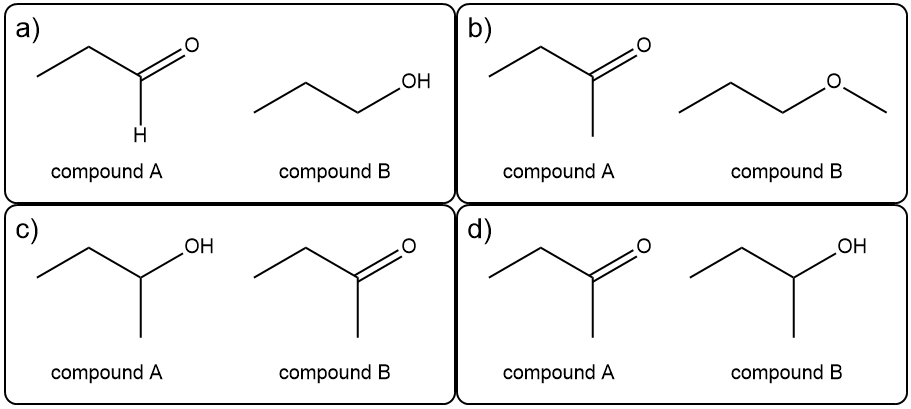
Determine the structure of the compounds.
IR absorptions at 1630 cm−1 and 1685 cm−1were observed for an unknown compound with molecular formula C4H5BrO. The proton NMR spectrum of the compound shows two doublets at 6.8 ppm and 7.5 ppm, respectively, with J = 15 Hz and one singlet at 2.3 ppm. Predict the structure consistent with the above information.
The spectrum information below is displayed as we see it in research journals. Draw the structure of the given data set.
C6H12O2: 1H NMR: δ 1.19 (6H, doublet), 1.21 (3H, triplet), 2.41 (2H, quartet), 4.93 (1H, septet); IR (cm-1): 1750, 1210
How can the following compounds be distinguished using only the techniques listed below and no other information?
a. using proton NMR spectroscopy
b. using 13C NMR with DEPT experiments.
c. using Infrared spectroscopy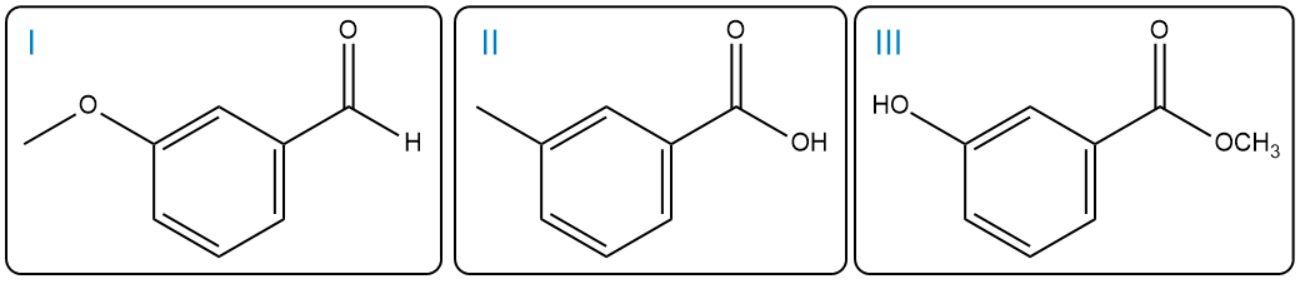
The 1H and 13C NMR spectrum of an unknown amine with the molecular formula C3H9N is given below. Propose the structure of this compound and show peak assignment for all the protons in it.
