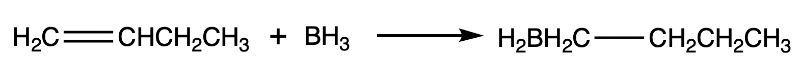- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Identify the reactants as Lewis acid (electrophile) and Lewis base (nucleophile) in the following reaction:

Indicate participating nonbonding electrons and draw curved arrows to describe how electrons move in this reaction.
Show the flow of electrons in each step of the following mechanism using curved arrows.

Consider the following reaction:

Identify the Lewis acid (electrophile) and Lewis base (nucleophile) in these reactions and draw curved arrows to indicate the flow of electrons.
Use curved arrows to show how the following nucleophiles react with the strong electrophile nitrosonium ion (NO+).
a. CH3CH2NH2
b. CH3O−
What are the types of arrows shown in the reaction? What are the products of the reaction?
Determine the type of each of the arrows marked in the following arrow-pushing mechanism.

Type I: nucleophilic attack by a lone pair of electrons
Type II: heterolytic cleavage of a σbond
Type III: nucleophilic attack by the π electrons
Type IV: dissociation of a π bond
Type V: formation of a π bond
In the following reaction, determine which is the nucleophile and which is the electrophile. Show the bond-making and bond-breaking processes using curved arrows.