Trace the path of an oxygen molecule in its journey from the air to a muscle cell in your arm, naming all the structures involved along the way.

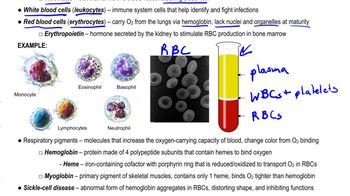
Mountain climbers often spend weeks adjusting to the lower partial pressure of oxygen at high altitudes before and during their ascent of high peaks. During that time, their bodies begin to produce more red blood cells. Some runners and cyclists prepare for competition by training at high altitudes or by sleeping in a tent in which PO₂ is kept artificially low. Explain why this training strategy may improve an athlete's performance.
 Verified step by step guidance
Verified step by step guidance
Verified video answer for a similar problem:
Key Concepts
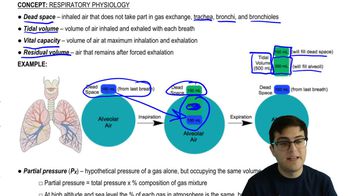
Partial Pressure of Oxygen (PO2)
Erythropoiesis
Altitude Training

Carbon monoxide (CO) is a colorless, odorless gas found in furnace and automobile engine exhaust and cigarette smoke. CO binds to hemoglobin 210 times more tightly than does O2. CO also binds with an electron transport protein and disrupts cellular respiration. Explain why CO is such a deadly gas.
Partial pressure reflects the relative amount of gas in a mixture and is measured in millimeters of mercury (mm Hg). Llamas are native to the Andes Mountains in South America. The partial pressure of O2 (abbreviated PO₂) in the atmosphere where llamas live is about half of the PO₂ at sea level. As a result, the PO₂ in the lungs of llamas is about 50 mm Hg, whereas that in human lungs at sea level is about 100 mm Hg. A dissociation curve for hemoglobin shows the percentage of saturation (the amount of O2 bound to hemoglobin) at increasing values of PO₂ As you see in the graph below, the dissociation curves for llama and human hemoglobin differ. Compare these two curves and explain how the hemoglobin of llamas is an adaptation to living where the air is 'thin.'
E-cigarettes pose a dilemma for public health officials. Because e-cigarettes produce fewer toxic chemicals than regular cigarettes, they may be a safer alternative for people who want to quit smoking but still crave nicotine. On the other hand, e-cigarettes may encourage nicotine addiction among teenagers. Evaluate the scientific evidence. Are e-cigarettes an effective aid for quitting cigarettes? What evidence supports the assertion that e-cigarettes are especially harmful to adolescents? The Centers for Disease Control website is a good place to start. cdc.gov/tobacco/basic_information/e-cigarettes/
